Depression is a leading cause of disability worldwide, with approximately 800,000 suicides occurring each year. There is a need for new antidepressant treatments that are safe, well-tolerated, rapid, long-lasting, and effective. Repetitive transcranial magnetic stimulation (rTMS) targeting the left dorsolateral prefrontal cortex (DLPFC) is a non-invasive brain stimulation technique that has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of treatment-resistant depression.

The FDA recently approved a more efficient form of repetitive transcranial magnetic stimulation (rTMS) called intermittent theta burst stimulation (iTBS). This new method shortens the treatment time from 37 minutes to just 3 minutes while producing equivalent antidepressant effects. Both FDA-approved rTMS and iTBS involve daily stimulation (600 iTBS pulses) for a total of 6 weeks. A study found that 32% of patients experienced symptom relief, and 49% showed a response to the treatment. Research indicates that the effectiveness of iTBS can be enhanced through accelerated and spaced delivery of stimulation, increasing the total number of pulses, precisely targeting the circuit between the left dorsolateral prefrontal cortex (DLPFC) and the subgenual anterior cingulate cortex (sgACC). Recent methodological advances suggest that the current iTBS protocols can be improved by:
- Administering multiple treatments daily with optimal intervals.
- Using a higher total number of stimulation pulses.
- Accurately targeting the DLPFC to sgACC circuit.
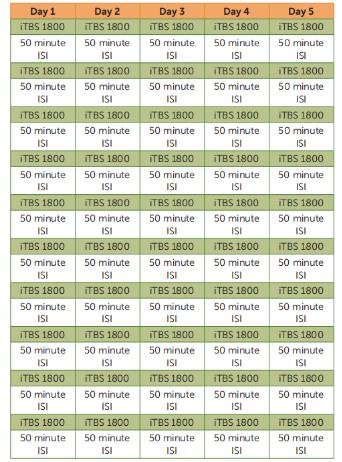
This study investigates the safety, tolerability, and preliminary efficacy of an accelerated high dose iTBS protocol targeting areas identified through functional connectivity MRI (fcMRI). The protocol involves 10 daily iTBS sessions (each delivering 1,800 pulses with a 50-minute interval between sessions) over five consecutive days (Monday to Friday), with stimulation targeting the left DLPFC region, which is functionally most anticorrelated with the sgACC. This protocol is referred to as Stanford Accelerated Intelligent Neuromodulation Therapy (SAINT). Our preliminary investigation into SAINT indicated efficacy in a small group of participants with severe and treatment-resistant depression, who were not included in this study.
The study recruited 23 participants, with 21 ultimately included. Among them, 19 were diagnosed with major depressive disorder, and 2 were diagnosed with bipolar II disorder, currently in a depressive episode (lasting more than one year).
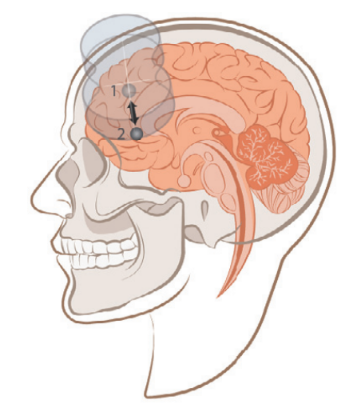
Before stimulation, each participant underwent structural MRI and resting-state functional MRI (fMRI) scans. Functional MRI was used to locate the left DLPFC region (Region 1), which has the most negative functional connectivity with the subgenual anterior cingulate cortex (sgACC, Region 2). This approach was chosen because previous neuroimaging studies have shown that the greater the negative correlation between the left DLPFC stimulation site and the sgACC, the better the clinical outcomes.
The FDA-approved iTBS protocol involves delivering 600 pulses once per day to the left dorsolateral prefrontal cortex (DLPFC) at 120% of the resting motor threshold, over a period of 6 weeks.
| Intensity | Burst Frequency(Hz) | BurstNumber | Train Frequency(Hz) | Trains number | Train Duration | ITI | Repetition Times | Total Pulses | Numbers of Session | |
| iTBS | 120%RMT | 50 | 3 | 5 | 10 | 2 | 8 | 20 | 600 | 6 weeks |
The SAINT protocol lasts for 5 days and delivers a pulse dose five times higher than the FDA-approved protocol. Participants receive one stimulation session per hour, with 10 sessions per day (18,000 pulses per day) for 5 consecutive days (a total of 90,000 pulses). The stimulation intensity is set at 90% of the resting motor threshold (RMT), with a safety limit not exceeding 120% RMT. Between sessions, participants wait in a designated area without interaction with researchers or other patients, to minimize interaction and prevent group effects. The choice of 1,800 pulses per session is based on previous blind iTBS trials and is the only dose explored in those studies. Moreover, 1,800 pulses have been shown to induce long-term changes in cortical excitability and optimally produce the desired cellular changes.
| Intensity | Burst Frequency (Hz) | BurstNumber | Train Frequency (Hz) | Trains number | Train Duration | ITI | Repetition Times | Total Pulses | |
| iTBS | 90%RMT | 50 | 3 | 5 | 10 | 2 | 8 | 60 | 1800 |
Depressive symptoms and suicidal ideation were evaluated both clinically and through self-reported measures before and after the SAINT treatment. The primary assessment tools used were the Hamilton Depression Rating Scale (HAMD), Montgomery-Åsberg Depression Rating Scale (MADRS), Columbia-Suicide Severity Rating Scale (C-SSRS; Suicidal Ideation Subscale), and Beck Depression Inventory-II (BDI-II). At the end of each day’s 10 stimulation sessions, depressive symptoms were assessed using a 6-item HAMD score. The Young Mania Rating Scale was completed daily to assess manic symptoms. Neuropsychological tests were conducted before and after SAINT to detect any neurocognitive side effects.
No serious adverse events occurred. The only side effects reported by participants were fatigue and some discomfort in the stimulation area and facial muscles during the sessions. Neuropsychological tests conducted after SAINT showed no negative cognitive side effects. In fact, significant improvements were observed in measures of cognitive inhibition, with no significant changes in any other neurocognitive tasks.
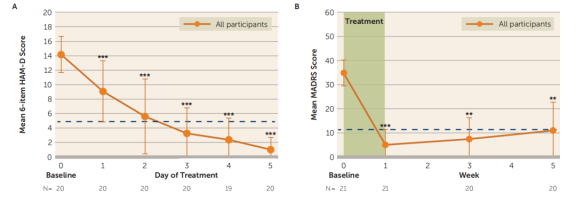
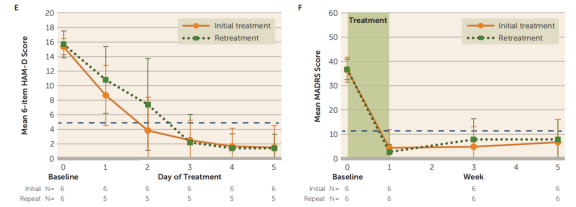
A generalized linear mixed model analysis revealed that the number of days had a significant impact on the average 6-item HAMD scores, and the number of weeks had a significant impact on the average MADRS scores. Scores at all follow-up time points were significantly lower than baseline levels.
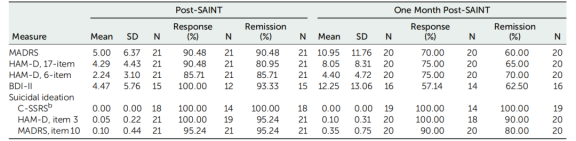
The results for the 17-item HAMD and BDI-II were summarized as well. The response rate (a ≥50% reduction in MADRS score from baseline) was 90.48%, with all responders achieving remission (MADRS score <11) after SAINT. One month after SAINT, 70% of participants continued to meet the response criteria.
The average number of days required to achieve a response (a ≥50% reduction in 6-item HAMD scores from baseline) with SAINT was 2.30 days (N = 20), and the average number of days to achieve remission (6-item HAMD score <5) was 2.63 days (N = 19; one participant did not reach remission). Kaplan-Meier survival analysis showed that participants who had not responded to the standard 6-week rTMS treatment (N = 6) required more days of treatment to reach a response with SAINT (average = 3.00 days).
Of the 21 participants, 19 reported some level of suicidal behavior on the C-SSRS during screening, 20 reported suicidal behavior on item 3 of the 17-item HAMD, and all 21 reported suicidal behavior on item 10 of the MADRS. After SAINT, scores on the C-SSRS, item 3 of the 17-item HAMD, and item 10 of the MADRS were significantly reduced at all follow-up time points. One month after SAINT, 80% to 100% of participants remained in remission on these measures.
Six participants who did not meet remission criteria but once again met the study's inclusion criteria underwent re-treatment (with an average interval of 20.5 weeks between treatments). The results showed no significant differences in daily 6-item HAMD scores or weekly MADRS scores between the initial and re-treatment courses. The daily 6-item HAMD scores and weekly MADRS scores at baseline and follow-up were similar during the re-treatment process.
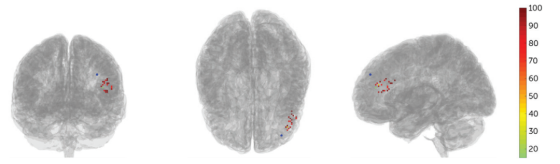
The results showed that MRI-guided neuronavigation provided more precise localization. The image below illustrates the individualized neuronavigation positioning of the left DLPFC compared to the F3 location using the 10-20 EEG system, with an average distance of 25.18mm between the two positions.
This study found that SAINT significantly reduced depressive symptoms and suicidal ideation in patients with treatment-resistant depression over a 5-day period, with no negative cognitive side effects observed. The remission rate observed in this study was higher compared to the FDA-approved standard rTMS protocol (37%), ECT (48%), and open-label remission rates for ketamine (31%) in treatment-resistant depression.
The high-dose, accelerated, fcMRI-guided iTBS protocol of SAINT appears to be initially safe and feasible, and is associated with a high rate of remission for depression. The potential efficacy of SAINT in treating suicidal ideation and the short duration of the protocol suggest that SAINT could provide a rapid method to ensure the safety of suicidal patients. However, larger, double-blind, placebo-controlled trials are needed to confirm the findings of this preliminary study.
The YINGCHI TMS devices M50 and M100, which include the TBS mode, are effective for treating treatment-resistant depression and can achieve high-efficiency treatment in a short period.

1.This content is organized by the Clinical Support Department of Shenzhen Yingchi Technology Co.,Ltd. Criticisms and corrections are welcome. For reprint, please indicate the source.
2.Reference:
Cole, E. J., Stimpson, K. H., Bentzley, B. S., Gulser, M., Cherian, K., Tischler, C., … Williams, N. R. (2020). Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression. American Journal of Psychiatry, appi.ajp.2019.1. doi: 10.1176/appi.ajp.2019.19070720