Release time :2025-12-15
Source:support@yingchitech.com
Scan:23

Major Depressive Disorder (MDD) is the leading cause of disability worldwide. Traditional medications and psychotherapy have limited efficacy in some patients. Although repetitive transcranial magnetic stimulation (rTMS) has been approved for MDD, the standard protocol has drawbacks including long treatment duration and slow onset of action.
To improve treatment efficiency, reduce costs, and increase accessibility, a Chinese research team has developed a novel “Sequential Dual-Target Accelerated rTMS” protocol. The results of this study were published in October 2025 in the top medical journal Cell Reports Medicine (Impact Factor = 10.6).
This was a single-center, double-blind, randomized, sham-controlled clinical trial (RCT), followed by a 6-month open-label follow-up phase.
A total of 52 outpatients with Major Depressive Disorder (MDD) meeting DSM-5 criteria (aged 18–55 years, MADRS score ≥20) were enrolled. Participants were randomly assigned in a 1:1 ratio to either the active stimulation group or the sham stimulation group. All participants were rTMS treatment-naïve, and antidepressant medication regimens were required to remain stable for at least 4 weeks prior to enrollment and throughout the entire study period.
Transcranial magnetic stimulation was delivered using the M-100 Ultimate device (Yingchi Technology, Shenzhen, China) with a liquid-cooled figure-8 coil (BY90A).
The left dorsolateral prefrontal cortex (dlPFC) was targeted using the “5-cm rule” (5 cm anterior to the motor hotspot). The dorsomedial prefrontal cortex (dmPFC) was targeted at 25% of the nasion–inion distance.
The primary clinical and imaging outcome measure was the Montgomery-Åsberg Depression Rating Scale (MADRS) score, with assessment time points including baseline, the day of treatment completion (Day 4), Week 2, and Week 4. Secondary outcome measures included the Hamilton Depression Rating Scale (HAMD-6), the Clinical Global Impression (CGI) scale, the Fatigue Severity Scale (FSS), and others. Furthermore, sham stimulation non-responders were offered active stimulation after Week 4, and all responders underwent up to 6 months of open-label follow-up. For imaging, resting-state functional magnetic resonance imaging (rsfMRI) data were acquired at baseline and 2 days after treatment completion.
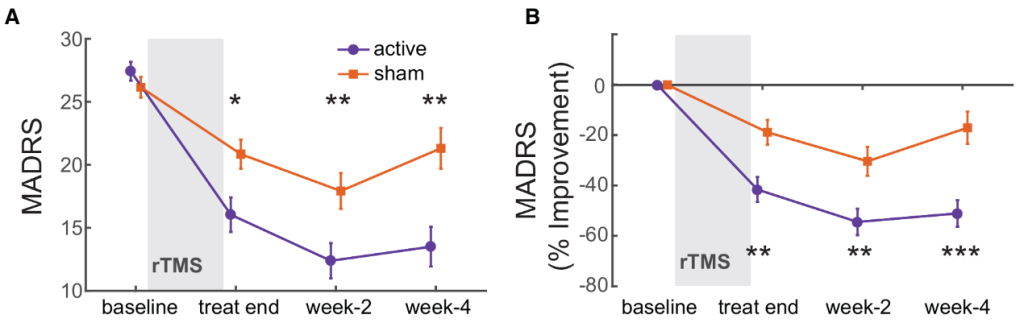
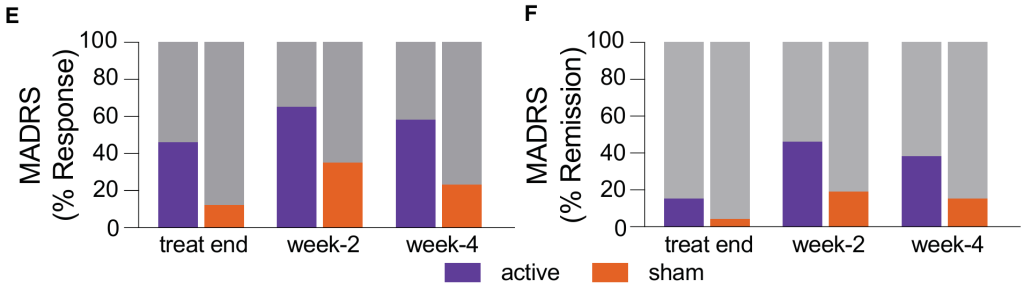
The MADRS scores showed a significant main effect of time (p=0.022) and a significant group-by-time interaction (F(1, 49)=13.197, p=0.001). By Day 4 after treatment completion, the MADRS score in the active stimulation group was already significantly lower than the sham stimulation group (mean difference = 4.704, p=0.016) , and this superiority persisted at Week 2 (p=0.014) and Week 4 (p=0.005). At Week 4 after treatment completion, the active stimulation group's average MADRS score reduction was 50.98%, which was significantly superior to the sham stimulation group; the active treatment group had a response rate of 57.69% and a remission rate of 38.46%; the sham stimulation group had a response rate of 23.08% and a remission rate of 15.38%.
Daily-rated HAMD-6 scores showed that the active stimulation group exhibited significant symptom improvement as early as Day 3. Furthermore, scores on the Fatigue Severity Scale (FSS) and the Apathy Evaluation Scale-Clinical Version (AES-C) also showed significant improvement in the active stimulation group.

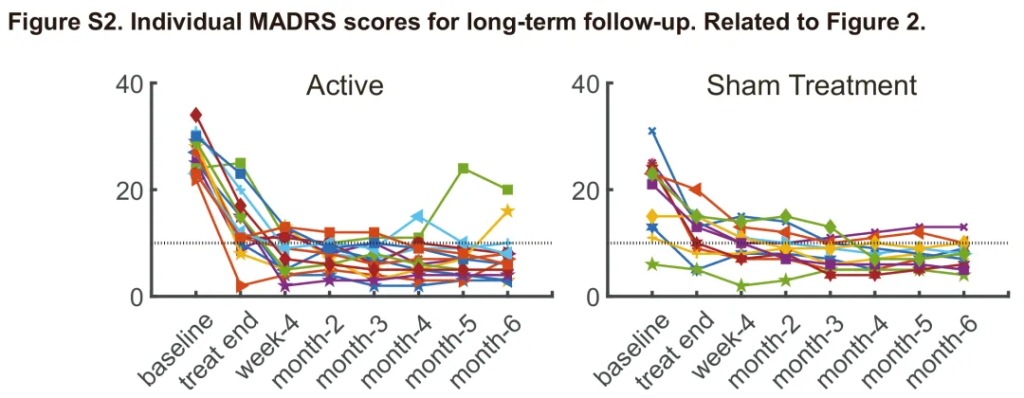
During the 6-month open-label follow-up, the efficacy demonstrated excellent stability for patients who achieved a treatment response at Week 4. The follow-up results showed that over 85% of responders maintained clinical remission (MADRS $\le 10$) within 6 months after treatment, with no significant trend of relapse.
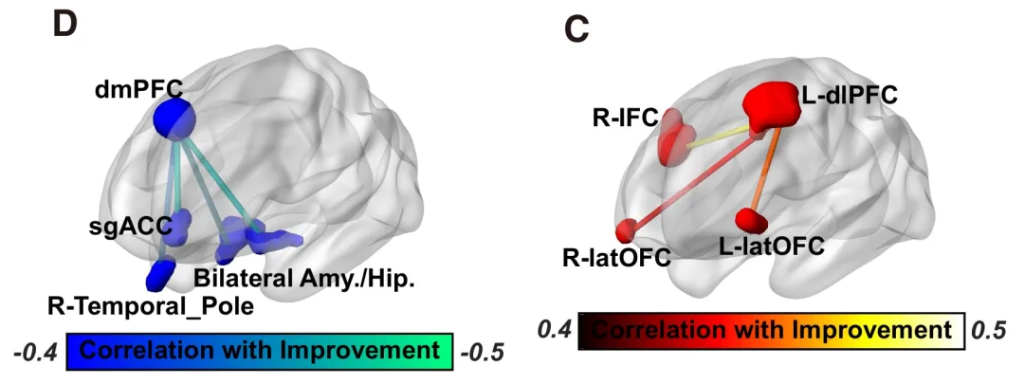
Resting-state fMRI analysis revealed a specific neural modulation mechanism for this protocol. Compared to the sham stimulation group, the active stimulation group showed dissociable changes in brain networks: the treatment significantly enhanced the functional connectivity (FC) between the left dorsolateral prefrontal cortex (dlPFC) and the frontoparietal network, and this enhanced connection was associated with improved cognitive control function. The treatment significantly reduced the functional connectivity between the dorsomedial prefrontal cortex (dmPFC) and the limbic system (including the amygdala, hippocampus, and subgenual anterior cingulate cortex [sgACC]), and this downregulation of connectivity was closely related to improved emotional regulation and the alleviation of depressive symptoms.
The treatment was generally well-tolerated, and all discomfort was transient and self-limiting. No seizures or other serious adverse events occurred during the study period.
This study proposed and validated an innovative dual-target accelerated rTMS protocol. The study utilized fMRI to elucidate the neurobiological basis for symptom improvement. This accelerated dual-target rTMS regimen demonstrated rapid and significant efficacy in treating Major Depressive Disorder (MDD), which may be attributed to several factors: Dual-Target Synergy and a "Priming Effect": The study employed a sequential stimulation strategy, stimulating the dlPFC first, followed by the dmPFC. This sequence may have contributed to the effectiveness of the rTMS treatment. The preceding stimulation of the dlPFC may have created a "pre-treatment" or "priming effect", enhancing the efficacy of the subsequent dmPFC stimulation by modulating the neural network state. Neuroimaging studies indicate that rTMS at the dlPFC can influence dmPFC activity. The authors' analysis also revealed a high degree of network overlap between the dlPFC and dmPFC, providing a neural basis for this priming interaction.
Previous research suggests that the cumulative effects of neuroplasticity persist for between 50 and 90 minutes. The authors emphasize the importance of setting the treatment interval to 50 minutes within the context of the dual-target accelerated rTMS protocol. Highly Efficient 20 Hz Stimulation Pattern: This study used a 3-minute 20 Hz stimulation sequence, which delivers a large number of pulses in a very short time, potentially not only enhancing efficacy but also accelerating the onset of the antidepressant effect.
This randomized controlled clinical trial demonstrates that the dual-target accelerated rTMS protocol sequentially targeting the left dlPFC and dmPFC is a safe, well-tolerated, and highly efficient treatment option for MDD. It achieves rapid and sustained antidepressant effects with a lower total pulse dose and less time commitment, offering a cost-effective and rapid new treatment alternative for patients with Major Depressive Disorder.

This content is organized by the Clinical Support Department of Shenzhen Yingchi Technology Co.,Ltd. Criticisms and corrections are welcome. For reprint, please indicate the source.
Reference:
Zhao, Y. J., Xiang, S., Chen, R., Ding, Q., Geng, R., Wang, Y., Li, Y., Li, H., Wang, Y., Cui, H., Huang, Y., Feng, J., Liu, W., & Voon, V. (2025). A sequential dual-site repetitive transcranial magnetic stimulation for major depressive disorder: A randomized clinical trial. Cell reports. Medicine, 6(10), 102402. https://doi.org/10.1016/j.xcrm.2025.102402